Fluorescence microscopy image formation
In bioimage analysis one often wants to quantify the intensities in an image. To do this properly one needs to be aware that these intensities are influenced by many factors, making intensity quantification in general very difficult. Sometimes the measured intensities can be affected so much that even object shape measurements can become difficult. For all those reasons it is very important to understand the reasons for signal distortion! Not knowing those effects can easily lead to wrong measurements.
Prerequisites
Before starting this lesson, you should be familiar with:
Learning Objectives
After completing this lesson, learners should be able to:
Understand how the intensities in a digital image that was acquired with a fluorescence microscope are formed
Understand how this image formation process has critical influence on the interpretation of intensity measurements
Understand that the geometry of your biological specimen can have a large influence on the measured intensities
Concept map
Figure
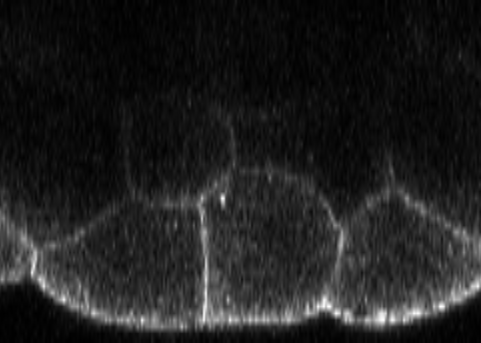
Activities
Confocal signal at membranes and inside a large specimen
- Open the zebrafish embryo confocal image
- Inspect the image along various axes and appreciate the following:
- Membranes appear with more contrast if they run along the optical axis
- Signal intensities become weaker to in the specimen’s interior, due to scattering and absorption
- Appreciate the cell segmentation (and morphometry) in the specimen interior are challenging/impossible
- Appreciate that intensity quantifications under such circumstances are highly challenging/impossible
Show activity for:
ImageJ GUI
- Open the mentioned image(s)
- Use
Plugins › BigDataViewer › Open Current Imageto inspect the image from different angles
- Use
Shift + Yto look along the y-axis- Use the mouse wheel to move along the y-axis
- Appreciate the mentioned effects on the membrane intensities
No optical sectioning in widefield microscopy
Depending on the microscopy method, one collects light from outside the current focal plane. In this activity, we will see this effect by comparing wide-field and confocal microscopy images of the same sample.
- Example data:
- Z-Stack with two “channels”, namely the same nucleus imaged with confocal and widefield fluorescence microscopy: xyzc_16bit__dna_conf_wf.tif
- Observe that a widefield image has the following properties:
- Depending on the z-position, different structures appear crisp
- However, the sum intensity in the image does (ideally) not depend in the z-position
- The latter has severe consquences for intensity measurements, namely wide-field microsopy is great to measure the total sum intensity of an object, but cannot be used to compare intensities at different z-positions
- This is very different from a confocal image, where the pinhole serves to only collect light at the current z-position
Show activity for:
ImageJ GUI
- Open the example image
- Place a ROI around the object in the image
- Use
Image › Stacks › Plot Z-axis Profileto measure the mean intensity at each z-position- Compute the change in intensity in percent from the brightest to the dimmest plane:
%change = 100% * (max - min) / (max - bg)
- Do this for both the confocal and the wide-field channel and compare the results
- Repeat, now using a much larger ROI such that all the blurred wide-field signal is always included in all z-planes
Assessment
Fill in the blanks
- In fluorescence microscopy the signal quality typically ___ when imaging deep inside a specimen.
- In confocal microscopy elongated structures that align with the z-axis typically appear ___ than elongated structures that align with the x or y-axis.
- In wide-field fluorescence microscopy there is no ___ and thus signal intensity quantifications for one specific z-position are typically not possible.
Solution
- decreases
- brighter
- optical sectioning
Follow-up material
Recommended follow-up modules:
Learn more: